Research Article |
|
Corresponding author: Volker W. Framenau ( volker.framenau@murdoch.edu.au ) Academic editor: Danilo Harms
© 2021 Volker W. Framenau, Renner L. C. Baptista, Francisca Sâmia M. Oliveira, Pedro de S. Castanheira.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Framenau VW, Baptista RLC, Oliveira FSM, Castanheira PS (2021) Taxonomic revision of the new spider genus Hortophora, the Australasian Garden Orb-weavers (Araneae, Araneidae). Evolutionary Systematics 5(2): 275-334. https://doi.org/10.3897/evolsyst.5.72474
|
Abstract
The new genus Hortophora in the orb-weaving spider family Araneidae Clerck, 1757 is established to include 13 species from the Australasian-Pacific region, with ten species known from Australia (five of which new to science): Hortophora biapicata (L. Koch, 1871), comb. nov. (type species) (= Araneus biapicatifera Strand, 1907, syn. nov.; = Epeira frosti Hogg, 1896, syn. nov.); H. cucullus sp. nov.; H. lodicula (Keyserling, 1887), comb. nov. (= Epeira scutigerens Hogg, 1900, syn. nov.); H. megacantha sp. nov.; H. porongurup sp. nov.; H. tatianeae sp. nov.; H. transmarina (Keyserling, 1865), comb. nov.) (also known from Papua New Guinea); H. urbana (Keyserling, 1887), comb. nov.; H. walesiana (Karsch, 1878), comb. nov. (= Epeira rhombocephala
Key Words
Taxonomy, systematics, new genus, Australia, backobourkiines
Introduction
Following their original descriptions in the mid- to late 1800s, few species of Australian orb-weaving spiders in the family Araneidae Clerck, 1757 have attracted detailed taxonomic attention (e.g.,
Amongst the few Australian araneid species taxonomically treated since their original description are two species commonly referred to as ‘Garden Orb-weavers’, Eriophora biapicata (L. Koch, 1871) and E. transmarina (Keyserling, 1865) (Fig.
Live images of Hortophora gen. nov. species. A, B. H. biapicata comb. nov., female from Hovea (Western Australia) (not collected); C. H. biapicata comb. nov., female from Rivervale (Western Australia) (WAM T100125); D, E. H. tatianeae sp. nov., female from East Ringwood (Victoria) (WAM T68055); F. H. tatianeae sp. nov., holotype male (Ringwood East, Victoria) (MV K-14612); G. H. transmarina comb. nov., female from Burbank (Qld) (WAM T84364). All images © V.W. Framenau.
A recent multigene molecular analysis of world-wide Araneidae also suggested that Nearctic and Australian Eriophora are only remotely related (
The aim of this study is to formally propose a new genus-group name for the Australian representatives of “NGEN01” (sensu
Material and methods
Descriptions and terminology follow recent publications on Australian backobourkiine orb-weaving spiders (e.g.,
Colour patterns are described based on specimens preserved in 75% ethanol. Male pedipalps were expanded by alternatively submerging it for 10 min in 10% KOH and distilled water until fully expanded. Redescriptions of historically named species are generally based on well preserved, recently collected material in lieu of often damaged and discoloured type specimens.
Throughout the course of this study that commenced in 2005, microscopic photographs were taken with two different stereo-imaging systems. A setup at the Natural History Museum of Denmark, Copenhagen, allowed taking images in different focal planes with a Nikon D300 digital SLR camera attached via a C-mount adapter from LM-Scope (http://www.lmscope.com) to a Leica M16A stereomicroscope. Images of different focal plains were stacked with Automontage (vers. 5.02) software from Syncroscopy to increase depth of field. We used 2 Nikon R1C1 wireless speedlights instead of fibre optics to illuminate the exposures. The latter were used as guide-light for focusing. A second set-up at the Harry Butler Institute, Murdoch University, Perth, supported taking microscopic images in different focal planes (ca. 20–30 images) on a Leica DMC4500 digital camera mounted to a Leica M205C stereomicroscope and combined using the Leica Application Suite X, v. 3.6.0.20104. Images of two type specimens lodged in the ZMH and NHM (Figs 39A–C, 40A, B) were taken with a basic digital camera and an adapter through the ocular tube of the microscopes in those institutions. All photos were edited and mounted with Photoshop CC 2020.
A male pedipalp of H. biapicata comb. nov. was prepared for scanning electron microscopy (SEM) imaging by passing it through a graded ethanol series of 70% to 100% and by subsequent critical point drying in a Baltec CPC-030 Critical Point Dryer. The specimen was then coated with Platinum-Palladium in a JEOL JFC-2300HR high resolution coater prior to scanning at 7kV in a JEOL JSM-6335F Field Emission Electron Microscope.
Maps were compiled in the software package QGis v. 2.14.0 Girona (https://qgis.org/en/site/; accessed 21 January 2020). Geographic coordinates were extracted directly from original labels or the registration data as provided by the museums. When no detailed geographic information was available, localities were estimated based on Google Earth v. 9.1.39.3 (https://earth.google.com/web/; accessed 21 January 2021).
All measurements are given in millimetres. They were taken with an accuracy of one tenths of a millimetre, with the exception of eye and labium measurements taken with an accuracy of one hundredth of a millimetre. The taxonomic part of this study lists all species in alphabetical order.
Abbreviations
Collections
MCZ Museum of Comparative Zoology, Harvard, USA;
MV Museums Victoria, Melbourne, Australia;
NTMAG Northern Territory Museum and Art Gallery, Darwin, Australia;
SAM South Australian Museum, Adelaide, Australia;
SMNH State Museum of Natural History, Stuttgart, Germany;
Morphology
ALE, AME anterior lateral (median) eyes;
PLE, PME posteriorposterior lateral (median) eyes.
Results
This study is a substantial contribution to resolving the taxonomy of the Australian Araneidae. Of a total of 11,723 examined records (= vials in collections) of Australian Araneidae in the research database of the senior author (VWF), 1,649 belong to the genus Hortophora gen. nov., totalling 2,208 specimens (455 males, 1,501 females and 252 juveniles) (Table
Species of Hortophora gen. nov., including information on primary types, distribution and number of records and specimens examined for this study. Abbreviations: ACT, Australian Capital Territory; NSW, New South Wales; NT, Northern Territory; Qld, Queensland; SA, South Australia; Tas, Tasmania; WA, Western Australia; m, males; f, females; j, juveniles.
| Species | Primary types, type locality, depository | Distribution | No. of records examined (no. of specimens) |
|---|---|---|---|
| Australian species | |||
| H. biapicata (L. Koch, 1871), comb. nov. | Holotype female, no exact locality (New Holland) (SMNH) (destroyed in WW2); Neotype male, 64 km W Westmar (Qld) ( |
ACT, NSW, NT, Qld, SA, Vic, WA | 647 (143m, 574f, 41j.) |
| H. cucullus sp. nov. | Holotype male, Pandappa Conservation Park (SA) (SAM NN19582) | NT, Qld, SA, WA | 20 (3m, 16f, 2j) |
| H. lodicula (Keyserling, 18887), comb. nov. | Holotype female, Sydney (NSW) ( |
ACT (inferred) NSW, Qld, Vic, Tas | 45 (10m, 48f, 30j) |
| H. megacantha sp. nov. | Holotype male, North Stradbroke Island (Qld) ( |
NSW, Qld | 27 (16m, 29f, 6j) |
| H. porongurup sp. nov. | Male holotype, Porongurup. National Park (WA) ( |
WA | 8 (5m, 6f, 6j) |
| H. tatianeae sp. nov. | Male holotype, East Ringwood (Vic) (MV K-14612) | ACT (inferred), NSW, Qld, SA, Tas, Vic | 274 (84m, 262f, 75j) |
| H. transmarina (Keyserling, 1865), comb. nov. | Unspecified number of female syntypes, NSW (no exact locality) (some specimens in |
NSW, NT, Qld, WA, also Papua New Guinea | 506 (149m, 483f, 79j) |
| H. urbana (Keyserling, 1887), comb. nov. | Male holotype, Sydney (NSW) (Bradley collection, considered lost) | NSW, Qld, WA | 95 (33m, 63f, 9j) |
| H. walesiana (Karsch, 1878), comb. nov. | Holotype male, New South Wales (no exact locality) ( |
NSW, NT, Qld, WA | 16 (4m, 11f, 1j) |
| H. yesabah sp. nov. | Male holotype, Dandabah, Bunya Mountains National Park (Qld) ( |
NSW, Qld | 11 (8m, 9f, 3j) |
| Pacific Island species | |||
| H. capitalis comb. nov. | Holotype female, Ovalau (Fiji) |
Fiji, New Caledonia, Vanuatu | 1(1f) |
| H. flavicoma comb. nov. | Holotype female from Canala (New Caledonia) (likely |
New Caledonia | - |
| H. viridis comb. nov. | Holotype female from Upolu (Samoa) ( |
Samoa | 1 (1f) |
We recognise a total of ten species of Hortophora gen. nov. from Australia, one of which also occurs in Papua New Guinea (Table
The centre of diversity of Hortophora gen. nov. is the east coast of Australia, specifically Queensland and New South Wales where nine of the ten Australian species occur; however, six species are also found in Western Australia, of which one, H. porongurup sp. nov., is endemic to the south-west of that state (Table
Taxonomy
Class Arachnida Cuvier, 1812
Order Araneae Clerck, 1757
Family Araneidae Clerck, 1757
Hortophora , gen. nov.
Type species
Epeira biapicata L. Koch, 1871 (designated here). Gender female.
Etymology
The generic name is composed of the stem hortus (Latin – garden), referring to the vernacular name of the species in Australia, Garden orb-weavers, and the ending –phora to indicate the similarity of the genus with Eriophora.
Diagnosis
Hortophora gen. nov. is here diagnosed against the only four genera of the backobourkiines (sensu
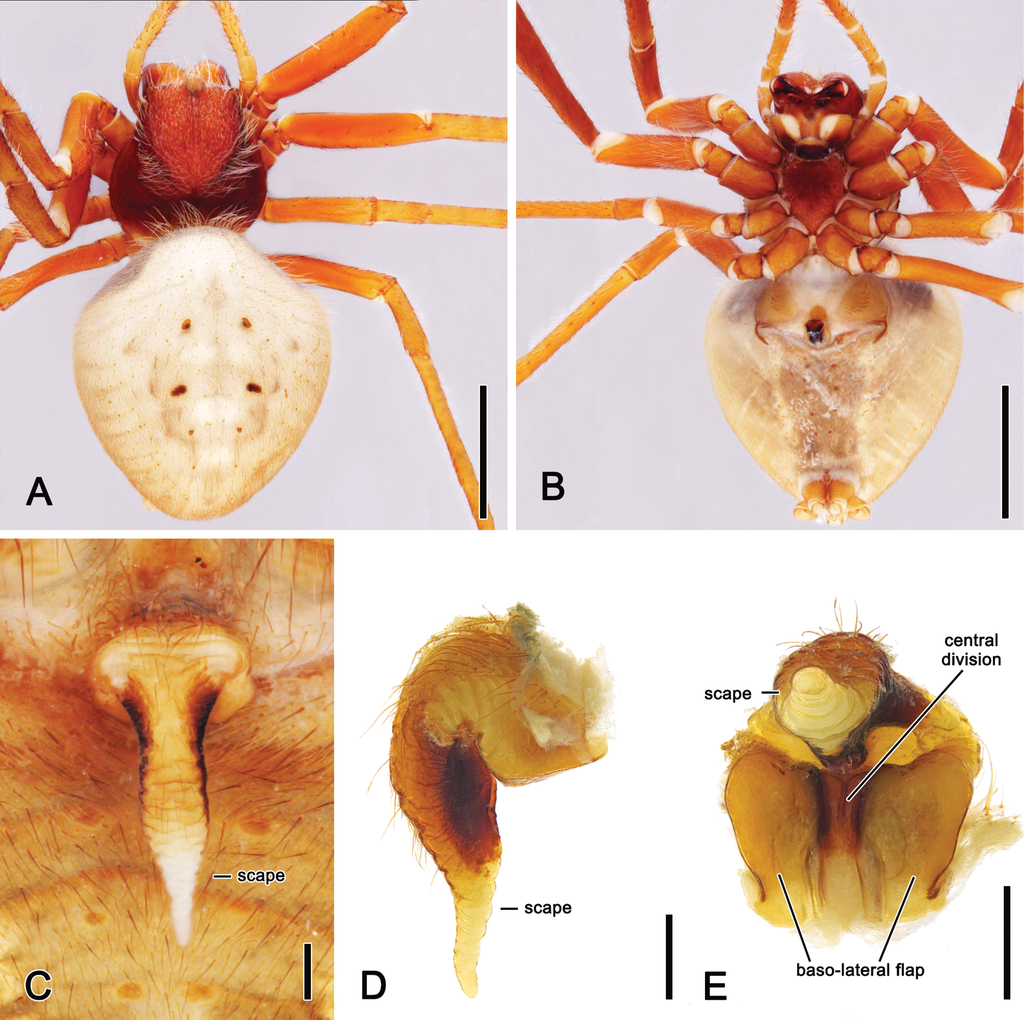
As we could not identify unambiguous synapomorphies of Hortophora gen. nov., we here propose the combination of the following characters to diagnose the genus within the backobourkiines: abdomen subtriangular to ovoid and generally with distinct humeral humps (Fig.
Hortophora gen. nov. species, second legs of male, prolateral view. A. H. biapicata comb. nov., right leg (
Hortophora gen. nov. differ from Backobourkia by the absence of a basal flange on the median apophysis of the male pedipalp and by the generally much longer, not elongate triangular epigyne scape of females. In addition, Hortophora gen. nov. species lack the characteristic anterior triangular white marking and the strong spine-like setae found on the dorsum of abdomen in Backobourkia.
Hortophora gen. nov., left male pedipalps, ventral view A. H. biapicata comb. nov. expanded (
Hortophora gen. nov. species differ from those of Plebs by an overall much larger body size, although large Plebs such as P. bradleyi (Keyserling, 1887) may overlap in size with smaller Hortophora gen. nov. Plebs species have a comparatively longer abdomen and Hortophora gen. nov. species lack the characteristic ventral abdominal pattern of Plebs, i.e. a squared, light Ü-pattern with the dots placed near the spinnerets. Most Hortophora gen. nov. species have indistinct lateral light lines on the ventral abdomen, sometimes with transverse light bands or patches (e.g., Fig.
Hortophora megacantha sp. nov., expanded right pedipalp (
The subtriangular abdomen of Hortophora gen. nov. greatly differs from the elongate abdomen of Lariniophora. Hortophora gen. nov. males lack the bilobed outgrowth on the median apophysis characteristic for Lariniophora, and females present an epigyne not as elevated and generally with a longer scape.
Male Hortophora gen. nov. differ from Novakiella by the elongate and transverse median apophysis of the pedipalp (short and pointing basally in Novakiella) and a comparatively smaller conductor lobe (heavily enlarged in Novakiella). In contrast to that of Novakiella females, the female epigyne base of Hortophora gen. nov. is rounded in ventral view and without wrinkles (triangular base with transverse or lateral wrinkles in Novakiella).
Hortophora biapicata comb. nov., scanning electron micrographs (SEMs) of right male pedipalp (
Description
Median to large-sized orb-weaving spiders, males (TL 5.9–11.5) generally smaller than females (TL 7.00–22.00). Carapace longer than wide, pear-shaped and with cephalic region relatively narrower in males than in females; colouration variable from beige to reddish-brown, often covered with dense white setae (e.g., Figs
Hortophora biapicata comb. nov. A. male dorsal habitus (
Male pedipalp patella with a single macroseta (e.g., Fig.
Hortophora biapicata comb. nov., male and female genitalia. A. left male pedipalp, ventral view (
Composition
13 species: H. biapicata comb. nov.; H. capitalis comb. nov.; H. cucullus sp. nov.; H. flavicoma comb. nov.; H. lodícula comb. nov.; H. megacantha sp. nov.; H. porongurup sp. nov.; H. tatianeae sp. nov.; H. transmarina comb. nov.; H. urbana comb. nov.; H. viridis comb. nov.; H. walesiana comb. nov.; H. yesabah sp. nov.
Distribution
Most species are restricted to Australia, with H. transmarina comb. nov. also found in Papua New Guinea (Table
Key to the species of Hortophora in Australia
Males
| 1 | Abdomen drawn out dorsally and arching posteriorly beyond the spinnerets, laterally with small humps (Fig. |
H. cucullus sp. nov. |
| – | Abdomen not drawn out dorsally and without lateral humps, subtriangular to ovoid | 2 |
| 2 | Coxa II with apico-ventral hook (Fig. |
H. biapicata comb. nov. |
| – | Coxa II without apico-ventral hook | 3 |
| 3 | Median apophysis of male pedipalp elongate transverse and ending in two (rarely one or three) apical, ventrally bent small hooks (Figs |
4 |
| – | Median apophysis ending in one (Figs |
6 |
| 4 | Median apophysis with distinct central protrusion (Figs |
5 |
| – | Median apophysis without central protrusion (Figs |
8 |
| 5 | Total length > 12 mm; tibia of leg II with more than 20 strong prolateral and ventral spines and apico-ventral spur indistinct (Fig. |
H. transmarina comb. nov. |
| – | Total length < 12 mm; tibia of leg II with less than 15 strong prolateral and ventral spines and apico-ventral spur distinct (Fig. |
H. tatianeae sp. nov. |
| 6 | Median apophysis ending in a single apical lobe, conductor lobe not extended apically (Figs |
7 |
| – | Median apophysis apically ending in two apical lobes, one of which being heavily sclerotised; conductor lobe extended apically (Fig. |
H. yesabah sp. nov. |
| 7 | Tip of terminal apophysis pointing towards base of pedipalp, in an almost 90 degree angle to embolus (Fig. |
H. walesiana comb. nov. |
| – | Tip of terminal apophysis pointing into the same direction as embolus (Fig. |
H. lodicula comb. nov. |
| 8 | Tibia of leg II with very large apico-ventral megaspur and spine (Fig. |
H. megacantha sp. nov. |
| – | Tibia of leg II without apico-ventral megaspur and spine (Fig. |
H. porongurup sp. nov. |
Females
| 1 | Abdomen slightly drawn out dorsally and with lateral protrusions (Fig. |
H. cucullus sp. nov. |
| – | Abdomen not drawn out apically without lateral protrusions | 2 |
| 2 | Shape of epigyne scape unknown (broken off in all specimens examined), but base of epigyne of very distinct shape in ventral and posterior views (Fig. |
H. porongurup sp. nov. |
| – | Epigyne base not with thick, sclerotised borders in ventral view and always with basally constricted central division in posterior view (e.g., Figs |
3 |
| 3 | Epigyne scape highly elongated, reaching by considerably more than half its length beyond the epigyne base (judged best in lateral view; Figs |
4 |
| – | Epigyne scape reaching at most by about half its length posteriorly beyond the epigyne base (Figs |
7 |
| 4 | Ventral abdomen with one or two broad, transverse light bands, particularly distinct just posterior of epigastric furrow (Figs |
5 |
| – | Ventral abdomen without transverse light bands, but with narrow light lateral bands and ca. three pairs of light spots medially (Figs |
6 |
| 5 | Baso-lateral flaps of epigyne enlarged, so that they are visible in ventral view (Fig. |
H. transmarina comb. nov. |
| – | Baso-lateral flaps of epigyne smaller and not visible in ventral view (Fig. |
H. biapicata comb. nov. |
| 6 | Central division of epigyne broad, ca. one third the width of the atrium in posterior view (Fig. |
H. megacantha sp. nov. |
| – | Central division of epigyne narrow, ca. one fifth of atrium (Fig. |
H. tatianeae sp. nov. |
| 7 | Epigyne scape not reaching beyond epigyne posteriorly (Fig. |
H. walesiana comb. nov. |
| – | Epigyne scape reaching beyond epigyne posteriorly (Figs |
8 |
| 8 | Epigyne scape comparatively broad and thick and widening centrally (Fig. |
H. urbana comb. nov. |
| – | Epigyne scape comparatively narrow and thin and of similar width along its length (Figs |
9 |
| 9 | Anteriorly directed base of scape rectangular (Fig. |
H. yesabah sp. nov. |
| – | Anteriorly directed base of scape triangular (Fig. |
H. lodicula comb. nov. |
Hortophora biapicata , comb. nov.
Epeira biapicata
Epeira frosti
Araneus biapicatifera
Araneus biapicatus
(L. Koch).-
Araneus biapicatifera
(Strand).-
Araneus frosti
(Hogg).-
Araneus transmarinus
(Keyserling).-
Eriophora biapicata
(L. Koch).-
Type material
Holotype
of Epeira biapicata L. Koch, 1871: female, “Neuholland” (= Australia; no precise locality data in original description) (SMNH) (lost in WW2, see
Neotype
of Epeira biapicata L. Koch, 1871 (designated by
Holotype of Epeira frosti Hogg, 1896: female, Stevenson River (27°06'S, 135°32'E, South Australia, Australia), Horn Expedition (MV K-931). Examined.
Holotype of Araneus biapicatifera Strand, 1907: female, no exact locality, only given as “Australien” in
Other material examined
See Appendix
Diagnosis
Male and female H. biapicata comb. nov. are most similar to H. transmarina comb. nov. with which they share a comparatively large size and distinct ventral pattern of broad light transverse bands of the abdomen, particularly distinct just behind the epigastric furrow (Figs
Description
Male (
Female (
Variation
Size variation: total length males 11.3–16.3 (n=17), females 15.0–23.1 (n=19). Unlike in other Hortophora gen. nov. species, the female scape was never broken off in any of the females examined by us. There is great colour variation, specifically in the abdomen of both males and females, which may be almost uniformly light to dark brown, or have a distinct folium pattern (e.g., Figs
The species was named after the often discernible two tips at the end of the abdomen in females. However, these cannot be seen in all specimens, in particular not in gravid or fully fed females and cannot be relied upon as diagnostic character.
Remarks
The holotype of Epeira biapicata L. Koch, 1871 was lost in WW2 when the Natural History Museum, Stuttgart was damaged.
Two immature females from Ovalau (Fiji) (
The female holotype of Epeira frosti Hogg, 1896 (MV K-931) agrees well with the diagnosis of H. biapicata comb. nov. as presented here. It is therefore proposed as junior synonym of H. biapicata comb. nov. Detailed images of the female holotype of Araneus biapicatifera Strand, 1907 (
Life history and habitat preferences
The large majority of mature males of H. biapicata comb. nov. has been found between January and March, with no records between August and October and very few in all other months. Mature females show an extended activity from January to May, with few records in all other months. Therefore, main reproductive activity of H. biapicata comb. nov. occurs in summer, extending into autumn for females. This coincides with the late wet season in northern Australia.
Eriophora biapicata comb. nov. can be found in almost all habitats that allow it to span its large orb-web between shrubs and trees. The species is also common in man-made environments, such as suburban parks and gardens.
Hortophora cucullus , sp. nov.
Type material
Holotype male, Pandappa Conservation Park (33°10'00"S, 139°08'15"E, South Australia, Australia), 22–25 April 2003, D. Hirst, night collection, mallee and sparse chenopods (SAM NN19582).
Etymology
The specific epithet is a masculine noun in apposition from Latin cucullus – a monk’s hat, and refers to the distinct abdominal shape, specifically of the male of this species.
Other material examined
See Appendix
Diagnosis
Males of Hortophora cucullus sp. nov. can be easily identified from all other Hortophora species by the lateral lobes on the abdomen of both males (Fig.
Hortophora cucullus sp. nov., male holotype (SAM NN19582). A. dorsal habitus; B. lateral habitus; C. ventral habitus; D. left pedipalp, ventral view; E. left pedipalp, dorsal view. Scale bars: 2 mm (A–C); 0.2 mm (D, E).
Description
Male (holotype, SAM NN19582): Total length 11.5. Carapace 4.1 long, 2.9 wide, dark brown with yellow setae mainly centrally (Fig.
Female (
Variation
Size variation: total length males 11.5–12.1 (n=2), females 13.1–17.8 (n=3). Little colour variation has been found within this species, although the abdomen may be a bit darker and less distinctly mottled than in the specimens illustrated here. No case of scape break-off was observed in female H. cucullus sp. nov.
Life history and habitat preferences
Mature males of H. cucullus sp. nov. were found between April and August suggesting reproductive activity mainly in winter (or the dry season in northern latitudes). Mature females were found between March and November, also suggesting that this species is not reproductively active in summer (or the wet season). The species has generally been found in open forests where the spiders build large orb-webs between shrubs and trees. Like H. biapicata sp. nov. it has been found in a variety of climatic conditions, including semi-arid to arid, tropical and temperate regions.
Distribution
Hortophora lodicula , comb. nov.
Epeira lodicula
Epeira scutigerens
Araneus lodiculus
(Keyserling).-
Araneus scutigerens
(Hogg).-
Type material
Holotype
of Epeira lodicula Keyserling, 1887: Female, Sydney (33°52'S, 151°12'E, New South Wales, Australia),
Syntypes
of Epeira scutigerens Hogg, 1900: 1 male, 1 female, Macedon (37°25'S, 144°33'E Victoria, Australia (
Other material examined
See Appendix
Diagnosis
The median apophysis of the pedipalp of male of H. lodicula comb. nov. is most similar to that of H. walesiana comb. nov. due to a broad apical lobe (Figs
Description
Male (
Female (
Variation
Size variation: total length males 7.5–10.6 (n=4), females 9.6–16.3 (n=9). The scape of the epigyne was not broken off in any females examined. Like other Hortophora gen. nov. species, the abdomen can be variable within the general folium pattern, and white guanine spots or lines are not uncommon. For example, the female syntype of E. scutigerens has a strong median guanine line along its whole abdomen (examined).
Remarks
The
The male and female syntypes of Epeira scutigerens Hogg, 1900 match in somatic and genitalic characters the diagnostic characters of H. lodicula comb. nov. and the species is therefore proposed as junior synonym of H. lodicula comb. nov.
Life history and habitat preferences
Mature males of H. lodicula comb. nov. have exclusively been found between January and April, suggesting this species to be summer- to autumn-mature. This matches the female phenology, as females appear somewhat earlier in the season, from November, and can be found into May. There is very little habitat information with specimens in collections, and these point to H. lodicula comb. nov. to inhabit open forests, including “amongst Proteas”.
Hortophora megacantha , sp. nov.
Type material
Holotype
male, Enterprise Mine, North Stradbroke Island (27°33'37"S, 153°27'06"E, Queensland, Australia),
Etymology
The specific epithet is a compound noun in apposition derived from the Ancient Greek mega (μέγας) – great, and acantha (Ἀκάνθα) – thorn, and refers to the large megaspur and spine on tibia of leg II in males.
Other material examined
See Appendix
Diagnosis
Males of Hortophora megacantha sp. nov. can be easily distinguished from all Hortophora by the presence of a large apico-ventral megaspur on the tibia of the second leg that is armed with a strong spine (Fig.
Description
Male (holotype,
Hortophora megacantha sp. nov., male holotype (
Female (
Variation
Size variation: total length males 5.6–7.5 (n=11), females 6.3–9.9 (n=16). The incidence of epigyne break-off was high in this species (ca. 75%), as only four of the 16 females measured had an intact scape. Colour pattern of the preserved specimens was fairly uniform as here described for the male and female with little major variation. Guanine patterns are prominent mainly in the anterior parts of the abdomen in some species.
Life history and habitat preferences
Mature males and females of H. megacantha sp. nov. can generally be found between November and February, with single records of males in April and September and a few records of females in April. Therefore, the species appears to be largely summer-mature. The species was found in a variety of forests and bushlands, including those with Blackbutt (Eucalyptus pilularis), and in vine thickets. Other habitat descriptions include mallee, scrubby gully and softwood scrub.
Hortophora porongurup , sp. nov.
Eriophora
sp. NGEN01 64:
Type material
Holotype
male, Porongurup National Park, S end of Millinup Pass (34°42'S, 117°54'E, Western Australia, Australia), M. S. Harvey, J. M. Waldock, 30 March 1993 (
Etymology
The specific epithet is a noun in apposition derived from the type locality, Porongurup National Park.
Other material examined
See Appendix
Diagnosis
Males of H. porongurup sp. nov. are easily identified by the extremely elongated median apophysis of the male pedipalp that reaches far beyond the pedipalp contour (Fig.
Description
Male (holotype,
Female (
Variation
Size variation: total length males 5.9–6.7 (n=3), females 7.0–8.8 (n=6). The scape of the female epigyne was broken off in all females examined. The folium pattern of H. porongurup sp. nov. with a dark central line is fairly consistent between specimens examined by us.
Life history and habitat preferences
Mature males and females of H. porongurup sp. nov. have only been found in March and April, suggesting this species to be autumn-mature. Only one specimen vial included a habitat description, which was ‘deep gully, in elevated leaf litter’.
Hortophora tatianeae , sp. nov.
Type material
Holotype male, Hume St, Ringwood East, unnamed park (37°49'26.88"S, 145°15'31.97"E, Victoria), 7 January 2019, V.W. Framenau, spotlighting (MV K-14612).
Etymology
The specific epithet is a matronym in apposition honouring Tatiane Almeida Diorio, wife of one of the junior authors (PSC), for her support during his research career.
Other material examined
See Appendix
Diagnosis
Male and female genital morphology of H. tatianeae sp. nov. is most similar to H. biapicata comb. nov. and H. transmarina comb. nov., but differs from both species in the distinctly different ventral abdomen colouration that lacks the broad transverse light bands (Fig.
Description
Male (holotype, MV K-14612): Total length 7.8. Carapace 4.5 long, 3.5 wide, centrally beige and with dark brown lateral flanks, white setae particularly centrally (Fig.
Hortophora tatianeae sp. nov., male holotype (MV K-14612). A. dorsal habitus; B. ventral habitus; C. left pedipalp, ventral view; D. left pedipalp, dorsal view. Scale bars: 2 mm (A, B); 0.5 mm (C, D).
Female (MV K-14613): Total length 10.1. Carapace 4.5 long, 3.9 wide; reddish-brown with darker lateral flanks (Fig.
Variation
Size variation: total length males 6.3–11.3 (n=21), females 6.3–14.0 (n=31). There was no incidence of epigyne scape breaking in H. tatianeae sp. nov. in any of the specimens examined by us. Dorsal abdominal colour variations are similar to that of H. biapicata comb. nov. and H. transmarina comb. nov., which range from a fairly uniform light to dark brown colour, faint to distinct folium pattern as described here and variable guanine patterns, as for example in the holotype male (Fig.
Life history and habitat preferences
Mature males of H. tatianeae sp. nov. have been found from December to April, with a single record in May. Mature females have been found throughout the year, but with very low numbers between June and November. Therefore, the species is largely summer- to autumn mature. Habitat descriptions include open, dry sclerophyll and rainforest, but the species has also been found in urban parks and gardens.
Hortophora transmarina , comb. nov.
Epeira transmarina
Epeira producta
Not Epeira transmarina Keyserling sensu
Araneus productus
(L. Koch).-
Araneus transmarinus
(Keyserling).-
Not Aranea producta (L. Koch) sensu
Eriophora producta (L. Koch).- Archer, 1951: 21.
Eriophora transmarina
(Keyserling).-
Araneus transmarinus
(Keyserling).-
Not Eriophora transmarina (Keyserling) sensu
Type material
Syntypes
of Epeira transmarina Keyserling, 1865: Based on original description an unknown number of females, New South Wales (no exact locality), Dr Graeffe leg., Museum Godeffroy (today largely in
Syntypes of Epeira producta L.
Other material examined
See Appendix
Diagnosis
Males of H. transmarina comb. nov. are, due to the ventral colour pattern and similar body-size, most similar to H. biapicata comb. nov. However, they differ from those of H. biapicata comb. nov. by the absence of coxal hooks on leg II (Fig.
Description
Male (
Hortophora transmarina comb. nov., male (
Female (
Variation
Size variation: total length males 12.2–16.3 (n=10), females 18.1–25.8 (n=7). There was no incidence of a broken scape in the material of H. transmarina comb. nov. examined by us. Abdominal colour patterns varied in similar fashion as in H. biapicata comb. nov. and H. tatianeae sp. nov. with almost uniformly very dark brown specimens to those with distinct folium pattern and a variety of white guanine patterns.
Remarks
There is ample confusion about the type material of Epeira transmarina Keyserling, 1865 and Epeira producta L. Koch, 1867 which we have tried to resolve here based on an exhaustive examination of material in all institutions where Australian types of these authors are mainly known from, i.e. Hamburg (
Epeira transmarina was described based on multiple (in German “mehrere”) (i.e., unknown number of) syntypes collected in New South Wales (
The
Ludwig
Life history and habitat preferences
Mature males of H. transmarina comb. nov. have largely been found between December and March, with few records in other months, but none in September and October. Mature females were found largely between December and May, with very few records between June to November. Therefore, the species is largely summer mature (or in the northern latitudes wet season). Within its range, H. transmarina comb. nov. is found in open woodlands, from dry sclerophyll to rainforest, wherever it can fix its large orb-webs between shrubs and trees. It is also common in suburban parks and gardens.
Distribution
Hortophora transmarina comb. nov. has been found along the east coast of Australia from southern New South Wales to the Top End, but also into the northern parts of the Northern Territory and Western Australia (Fig.
Hortophora urbana , comb. nov.
Epeira urbana
Araneus urbanus
(Keyserling).-
Type material
Holotype
of Epeira urbana Keyserling, 1887: Male, Sydney (33°52'S, 151°12'E, New South Wales, Australia), Bradley collection (considered lost; see
Other material examined
See Appendix
Diagnosis
Males of H. urbana comb. nov. can easily be distinguished from all other Hortophora gen. nov. species by distinct shape of the median apophysis of male pedipalp. Whilst it is elongate transverse with a central protrusion as in many other species, both the central protrusion and the apical tip are blunt with only very small teeth apically (Figs
Description
Male (
Female (
Variation
Size variation: total length males 10.0–13.1 (n=4), females 13.1–20.0 (n=8). The epigyne scape was broken off in half the females measured here. Abdominal colour patterns are fairly variable in H. urbana comb. nov. male and females, from dark specimens to very light ones as illustrated here. The folium pattern is generally not very distinct, but light guanine spot and lines are frequent.
Remarks
The holotype of Epeira urbana Keyserling, 1887 was part of the Bradley collection and is considered lost (
We described the best preserved female available to us, but as the scape of this specimens was broken off (Fig.
Life history and habitat preferences
Mature males of H. urbana comb. nov. were collected in November and December and mature females from December to April indicating summer-maturity (equivalent to the wet season in northern latitudes). Habitat descriptions with museum specimens include rainforest and softwood scrub, but a single record is from a suburban clothes line.
Hortophora walesiana , comb. nov.
Epeira walesiana
Epeira rhombocephala
Cyclosa rhombocephala
(Thorell).-
Epeira lutulenta
Type material
Holotype
of Epeira walesiana Karsch, 1878: Male, New South Wales (no exact locality), Daemel (
Holotype
of Epeira rhombocephala Thorell, 1881: Male, Somerset, Cape York (10°43'S, 142°31'E, Queensland, Australia) 1875, L. D’Albertis (
Holotype
of Epeira lutulenta Keyserling, 1886: Female, Peak Downs (22°56'S, 148°05'E, Queensland, Australia) (
Other material examined
See Appendix
Diagnosis
Males of H. walesiana comb. nov. are most similar to those of H. lodicula comb. nov. due to the comparatively short median apophysis of the pedipalp that terminates in an apically pointing lobe. (Fig.
Description
Male (
Hortophora walesiana comb. nov., male (
Female (
Variation
Size variation: total length males 5.2–6.4 (n=15), females 8.2–10.1 (n=6). We did not observe any scape break-off in H. walesiana comb. nov. Colour variations are as reported for other Hortophora gen. nov. species, from fairly dark (as in the male described here) to the light colouration of the female illustrated, but the folium pattern is generally fairly indistinct.
Remarks
Somatic and genitalic characters, specifically male pedipalp morphology of the holotype of Epeira rhombocephala Thorell, 1881 match H. walesiana comb. nov. as diagnosed here. Epeira rhombocephala is therefore proposed as junior synonym of H. walesiana comb. nov. Similarly the female holotype of Epeira lutulenta Keyserling, 1886 matches in somatic and genitalic morphology those belonging to H. walesiana comb. nov. and therefore Epeira lutulenta is proposed as junior synonym of H. walesiana comb. nov.
Life history and habitat preferences
Mature males have been found in January and February, with a single record from June. Mature females have been found from January to April, with a single record in August. Hortophora walesiana comb. nov. therefore appears to be most active in the late dry season, considering that the species is limited to the northern half of the country.
Distribution
Hortophora walesiana comb. nov. has been found mainly towards the coastal areas in the northern half of Australia, north of ca. 27°S Latitude in the Northern Territory, Queensland and Western Australia (Fig.
Hortophora yesabah , sp. nov.
Type-material
Holotype
male, Dandabah, Bunya Mountains National Park (26°51'S 151°34'E, Queensland, Australia),
Etymology
The specific epithet refers to the Yesabah Caves (New South Wales), one of the few localities where the species was found. It is a noun in apposition.
Other material examined
See Appendix
Diagnosis
The male pedipalp of male H. yesabah sp. nov., specifically the shape of the median apophysis is unlike any other in the genus, as it terminates in two large somewhat pointy lobes, of which the dorsal one is heavily sclerotised (Fig.
Description
Male (holotype,
Female (
Variation
Size variation: total length males 7.5–8.9 (n=4), females 8.6.1–10.9 (n=5). The epigyne scape was broken off in one of five females measured for this study. A folium pattern is always clearly discernible in all specimens of H. yesabah sp. nov. examined by us, but no distinct white guanine patterns were evident.
Life history and habitat preferences
Mature males of H. yesabah comb. nov. were found from February to July and mature females from March to October. This suggests that this species is autumn and winter mature. The only habitat description with collection specimens reads ‘rainforest’.
Non-Australian Hortophora gen. nov. species
Hortophora capitalis , comb. nov.
Epeira capitalis
Epeira capitalis
L. Koch.-
Araneus capitalis
(L. Koch).-
Type material
Holotype
of Epeira capitalis L. Koch, 1871: Female, from Ovalau (17°41'S, 178°48'E, Fiji)
Remarks
Hortophora capitalis comb. nov., holotype female from Ovalau (Fiji)
Hortophora flavicoma , comb. nov.
Epeira flavicoma
Araneus flavicoma
(Simon).-
Eriophora flavicoma
(Simon).-
Type material
Holotype of Epeira flavicoma Simon, 1880: Female, Canala (21°31'S, 165°57'E, New Caledonia), Coll. E. Simon (likely
Remarks
Epeira flavicoma Simon, 1880 was described based on a female from New Caledonia.
The detailed treatment of this species is not part of this project, but it is clear that
Hortophora viridis , comb. nov.
Epeira viridis
Type material
Holotype of Epeira viridis Keyserling, 1865: Female from Upolu (13°54'S, 171°44'E, Samoa) (
Remarks
Epeira viridis Keyserling, 1865 was synonymised with H. transmarina comb. nov. by
Hortophora viridis comb. nov. is much unlike H. transmarina comb. nov., specifically based on the shape and colouration of the abdomen and details of the epigyne (Fig.
Discussion
Hortophora gen. nov. includes largely Australian species with characteristic genital morphology. An elongated median apophysis with two apical, small protrusions is the most common male pedipalp configuration in the genus and occurs in those species with a very long epigyne scape. Amongst other backobourkiines with similar median apophyses, like for example Backobourkia and Plebs, the epigyne scape is likewise elongated (
Hortophora lodicula comb. nov., H. walesiana comb. nov. and H. yesabah sp. nov. have comparatively short median apophyses and epigyne scapes, particularly in H. walesiana gen. nov. The inclusion of these species in Hortophora gen. nov. may therefore be considered tentative pending a comprehensive morphological and molecular phylogenetic study of the backoubourkiine spiders (sensu
The backobourkiines are a largely Australian clade with few genera also occurring outside the country. Backobourkia, Novakiella and Lariniophora are Australian endemics, with the exception of two species, B. brounii (Urquhart, 1885) and N. trituberculosa (Roewer, 1942), that occur in New Zealand and may be human-mediated introductions there (
Solving biogeographic questions within Hortophora gen. nov. and the backobourkiines as a whole requires further taxonomic and phylogenetic studies. With respect to Hortophora gen. nov., future studies should concentrate on the Pacific Hortophora gen. nov., specifically the taxonomic treatment of males, and an evaluation of the phylogenetic position of Hortophora gen. nov. species with comparatively short median apophyses and epigyne scapes.
Acknowledgements
We foremost thank Nikolaj Scharff for hosting VWF on two occasions at the Zoological Museum, University of Copenhagen where many microscopy images, including SEMs, of this study were taken. The senior author also benefited greatly from discussions with Nikolaj on Araneidae taxonomy and systematics and homology of pedipalp sclerites. We thank (in no particular order) Peter Lillywhite, Joseph Schubert, Catriona McPhee, Ken Walker, Richard Marchant (NMV), Robert Raven, Owen Seeman, Wendy Hebron (
Funding for a revision of the Australian Araneidae was provided by the Australian Biological Resources Study (ABRS) (grant no. 205-24 [2005–2008] to VWF and N. Scharff and grant number 4-EHPVRMK [2021–2023] to VWF, PSC, N. Scharff, D. Dimitrov, A. Chopra and R. Baptista).
References
- Archer AF (1951) Studies in the orbweaving spiders (Argiopidae). 1. American Museum Novitates 1487: 1–52.
- Berland L (1924) Araigées de la Nouvelle-Calédonie et des Iles Loyalty. Nova Caledonia, Zoologie 3: 159–255.
- Berland L (1938) Araignées des Nouvelles-Hébrides. Annales de la Société Entomologique de France 107: 121–190.
- Castanheira P de S, Didham RK, Vink CJ, Framenau VW (2019) The scorpion-tailed orb-weaving spiders (Araneae: Araneidae: Arachnura) in Australia and New Zealand. Zootaxa 4706: 147–170. https://doi.org/10.11646/zootaxa.4706.1.6
- Chrysanthus F (1960) Spiders from South New Guinea III. Nova Guinea, Zoology 3: 23–42.
- Davies VT (1980) Two large Australian orb-weaving spiders, Eriophora transmarina (Keyserling 1865) and Eriophora biapicata (L. Koch 1871). Memoirs of the Queensland Museum 20: 125–133.
- Davies VT (1988) An illustrated guide to the genera of orb-weaving spiders in Australia. Memoirs of the Queensland Museum 25: 273–332.
- Dondale CD (1966) The spider fauna (Araneida) of deciduous orchards in the Australian Capital Territory. Australian Journal of Zoology 14: 1157–1192. https://doi.org/10.1071/ZO9661157
- Framenau VW (2005) The wolf spider genus Artoria Thorell in Australia: new synonymies and generic transfers (Araneae, Lycosidae). Records of the Western Australian Museum 22: 265–292. https://doi.org/10.18195/issn.0312-3162.22(4).2005.265-292
- Framenau VW (2011) Lariniophora, a new monotypic orb-weaving spider genus from Australia (Araneae: Araneidae: Araneinae). Records of the Western Australian Museum 26: 191–201. https://doi.org/10.18195/issn.0312-3162.26(2).2011.191-201
- Framenau VW (2019) Generic and family transfers, and nomina dubia for orb-weaving spiders (Araneae, Araneidae) in the Australasian, Oriental and Pacific regions. Evolutionary Systematics 3: 1–27. https://doi.org/10.3897/evolsyst.3.33454
- Framenau VW, Dupérré N, Blackledge TA, Vink CJ (2010) Systematics of the new Australasian orb-weaving spider genus Backobourkia (Araneae: Araneidae: Araneinae). Arthropod Systematics and Phylogeny 68: 79–111.
- Framenau VW, Scharff N, Vink CJ, Baptista RLC, Castanheira P de S (2021) Review of the Australian and New Zealand orb-weaving spider genus Novakiella (Araneae: Araneidae). Zoosystematics and Evolution 97: 393–405. https://doi.org/10.3897/zse.97.67788
- Hogg HR (1896) Araneidae. In: Spencer B (Ed.) Report of the Horn Expedition to Central Australia. Pt. 2. Zoology. Melville, Mullen and Slade, Melbourne, 309–356.
- Hogg HR (1899) Notes on some spiders from the Upper Endeavour River, Queensland, with description of two new species. Proceedings of the Royal Society of Victoria 11: 137–146.
- Hogg HR (1900) A contribution to our knowledge of the spiders of Victoria; including some new species and genera. Proceedings of the Royal Society of Victoria 13: 68–123.
- Joseph MM, Framenau VW (2012) Systematic review of a new orb-weaving spider genus (Araneae: Araneidae), with special reference to the Australasian-Pacific and South-East Asian fauna. Zoological Journal of the Linnean Society 166: 279–341. https://doi.org/10.1111/j.1096-3642.2012.00845.x
- Kallal RJ, Hormiga G (2018) Systematics, phylogeny and biogeography of the Australasian leaf-curling orb-weaving spiders (Araneae: Araneidae: Zygiellinae), with a comparative analysis of retreat evolution. Zoological Journal of the Linnean Society 184: 1055–1141. https://doi.org/10.1093/zoolinnean/zly014
- Kallal RJ, Fernández R, Giribet G, Hormiga G (2018) A phylotranscriptomic backbone of the orb-weaving spider family Araneidae (Arachnida, Araneae) supported by multiple methodological approaches. Molecular Phylogenetics and Evolution 126: 129–140. https://doi.org/10.1016/j.ympev.2018.04.007
- Karsch F (1878) Exotisch-araneologisches. Zeitschrift für die gesamten Naturwissenschaften 51: 322–333, 782–826.
- Keyserling E (1865) Beiträge zur Kenntniss der Orbitelae Latr. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 15: 799–856.
- Keyserling E (1886) Die Arachniden Australiens nach der Natur beschrieben und abgebildet. 2. Theil. 2. Lieferung. Bauer & Raspe, Nürnberg, pg. 87–152, pl. 7–12.
- Keyserling E (1887) Die Arachniden Australiens nach der Natur beschrieben und abgebildet. 2. Theil. 3. Lieferung. Bauer & Raspe, Nürnberg, pg. 153–232, pl. 12–20.
- Koch L (1867) Beschreibungen neuer Arachniden und Myriapoden. II. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 17: 173–250.
- Koch L (1871) Die Arachniden Australiens nach der Natur beschrieben und abgebildet. 1. Theil. 1. Lieferung. Verlag von Bauer und Raspe, Nürnberg, pg. 1–104, pl. 1–8. https://doi.org/10.5962/bhl.title.121660
- Levi HW (1971) The ravilla group of the orbweaver genus Eriophora in North America (Araneae: Araneidae). Psyche 77: 280–302. https://doi.org/10.1155/1970/69275
- Levi HW (1972) The orb-weaver genera Singa and Hypsosinga in America (Araneae: Araneidae). Psyche, Cambridge 78: 229–256. https://doi.org/10.1155/1971/76327
- Levi HW (1983) The orb-weaver genera Argiope, Gea, and Neogea from the Western Pacific Region (Araneae: Araneidae, Argiopinae). Bulletin of the Museum of Comparative Zoology 150: 247–338.
- Levi HW (1992) The American species of the orb-weaver genus Carepalxis and the new genus Rubrepeira (Araneae: Araneidae). Psyche, Cambridge 98: 251–264. https://doi.org/10.1155/1991/26493
- Lise AA, Kesster CC, Silva ELC da (2015) Revision of the orb-weaving spider genus Verrucosa McCook, 1888 (Araneae, Araneidae). Zootaxa 3921: 1–105. [incl. Erratum: Zootaxa 3956(4): 600] https://doi.org/10.11646/zootaxa.3921.1.1
- Main BY (1964) Spiders of Australia. A guide to their identification with brief notes on the natural history of common forms. Jacaranda Press, Sydney, NSW.
- Mouginot P, Prügel J, Thom U, Steinhoff POM, Kupryjanovicz J, Uhl G. (2015) Securing paternity by mutilating female genitalia in spiders. Current Biology 25: 2980–2984. http://doi.org/10.1016/j.cub.201509.074
- Mouginot P, Uhl G (2019) Females of cannibalistic spider control mutilation of their genitalia by males. Behavioral Ecology 30: 1624–1631. https://doi.org/10.1093/beheco/arz127
- Musgrave A (1933) The Garden Orb-waving Spider. Australian Museum Leaflet 5, Sydney, Australia.
- Nakata K (2016) Female genital mutilation and monandry in an orb-web spider. Biology Letters 12: 20150912. https://doi.org/10.1098/rsbl.2015.0912
- Rack G (1961) Die Entomologischen Sammlungen des Zoologischen Staatsinstituts und Zoologischen Museums Hamburg. II. Teil. Chelicerata II: Araneae. Mitteilungen des Hamburgischen Zoologischen Museums und Instituts 59: 1–60.
- Rainbow WJ (1909) Notes on the architecture, nesting habits and life histories of Australian Araneidae, based on specimens in the Australian Museum. Part VII. Entelegynae (continued). Records of the Australian Museum 7: 212–234. https://doi.org/10.3853/j.0067-1975.7.1909.963
- Rainbow WJ (1911) A census of Australian Araneidae. Records of the Australian Museum 9: 107–319. https://doi.org/10.3853/j.0067-1975.9.1911.928
- Scharff N, Coddington JA, Dimitrov D, Agnarsson I, Framenau VW, Szüts T, Blackledge TA (2020) Phylogeny of the orb-weaving spider family Araneidae (Araneae, Araneoidea). Cladistics 36: 1–21. https://doi.org/10.1111/cla.12382
- Schmeltz JDE (1866) Catalog III der zum Verkauf stehenden Doubletten aus den naturhistorischen Expeditionen der Herren Joh. Ces. Godeffroy & Sohn in Hamburg. [no publisher given], Hamburg.
- Schmeltz JDE (1869) Museum Godeffroy. Catalog IV, nebst einer Beilage, enthaltend: Topographische Notizen; Beschreibung neuer Bryozoen von Senator Dr. Kirchenpauer zu Hamburg und einer neuen Asteriden-Gattung von Dr. Chr. Lütken zu Kopenhagen. Wilhem Mauke Söhne, vormals Perthes-Besser & Mauke, Hamburg.
- Simon E (1880) Matériaux pour servir à une faun arachnologique de la Nouvelle Calédonie. Annales de la Société Entomologique de Belgique 23 (C.R.): 164–175.
- Simon E (1895) Histoire Naturelle des Araignées. Deuxième édition. Tome premier. Fascicule 4. Librairie Encyclopédique de Roret, Paris.
- Strand E (1907) Einige Spinnen aus Kamerun, Java und Australien. Jahrbuch des nassauischen Vereins für Naturkunde 60: 177–219.
- Strand E (1913) Über einige australische Spinnen des Senckenbergischen Museums. Zoologische Jahrbücher für Systematik 35: 599–624. https://doi.org/10.5962/bhl.part.16722
- Thorell T (1881) Studi sui Ragni Malesi e Papuani. III. Ragni dell’Austro-Malesia e del Capo York, conservati ne Museo Civico di Storia Naturale die Genova. Annali del Museo Civico di Storia Naturale di Genova 17: 1–727.
- World Spider Catalog (2021) World Spider Catalog. Version 22.0. Natural History Museum Bern, Bern, Switzerland. [Available at:] http://wsc.nmbe.ch [Accessed 12 July 2021] https://doi.org/10.24435/2
Appendix 1
Material examined of Hortophora gen. nov.
Hortophora biapicata comb. nov.
Material examined. Australia: Australian Capital Territory: 1 female, Canberra, 35°18'S, 149°08'E (
Hortophora cucullus sp. nov.
Material examined: Australia: No exact location: 1 female, labelled “Camp 5”, no exact locality (NMV K-10371); Northern Territory: 1 female, Goyder Creek, 25°40'S, 134°25'E (
Hortophora lodicula comb .nov.
Material examined: Australia: New South Wales: 1 female, Barren Grounds Reserve (
Hortophora megacantha sp. nov.
Material examined: Australia: New South Wales: 1 male, Beecroft Reserve, 33°45'S, 151°04'E (
Hortophora porongurup sp. nov.
Australia: Western Australia: 2 females, 4 juv., Dog Pool, Shannon National Park, 34°46'S, 116°22'E (
Hortophora tatianeae sp. nov.
Material examined: Australia: New South Wales: 1 male, Arcadia, 33°37'S, 151°03'E (
Hortophora transmarina comb. nov.
Material examined: Australia: New South Wales: 1 female, 3 juv., no exact location (
Hortophora urbana comb. nov.
Australia: New South Wales: 1 female, Bayles Creek, Cheltenham, 33°45'S, 151°05'E (
Hortophora walesiana comb. nov.
Material examined. Australia: Northern Territory: 1 female, Melville Island, 17 Mile Plains, 11°33'S,130°56'E (NTMAG). Queensland: 1 female, Bruce Highway, 4 km S Bowen, 20°02'S, 148°14'E (NMV K-10368); 1 female, Camp Milo, Cooloola, 26°S, 153°05'E (
Hortophora yesabah sp. nov.
Australia: New South Wales: 1 female, Jamberoo Mountain, 34°40'S, 150°43'E (